Menu
Physics Lesson 20.2.1 - Nuclear Force
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Nuclear Force, this is the first lesson of our suite of physics lessons covering the topic of Nuclear Forces, Defect of Mass and Binding Energy, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Nuclear Force
Since in any atomic nucleus there are Z positively charged protons, it is evident that an attracting force must exist to keep the nucleons held together (i.e. to keep the nucleus stable), as opposed to repelling electric force caused by like charged protons. This force known as nuclear force, must balance the electric force acting between protons. Obviously, in order to perform its function (i.e. to keep nucleons inside the nuclei), nuclear force must act in very short distances, not more than 10-15 m, as it must not exceed the dimensions of atomic nuclei.
Despite nucleons may move in respect to each other, there is a constant equilibrium between the above two forces, otherwise nucleons would collide with each other. Experiments show that the net force between nucleons is more repulsive at short distances (electric force is greater than nuclear force) and more attractive at medium distances (nuclear force is greater than electric force). In other words, there are two forces acting between nucleons: one is the electric (repulsive) force and the other is the nuclear (attractive) one. The distance between nucleons determines which of these forces overcomes the other. Logically, there must be an equilibrium position in which the nucleus acquires stability and where the magnitudes of the above two forces are equal (the net force is zero).
As noted above, the two forces (attractive and repulsive) act at distances smaller than the dimensions of nuclei. For bigger distances, both forces - especially the attracting (nuclear) force we are concerned with in this article - point towards zero. The nuclear force vs distance correspondence is given in the graph below.
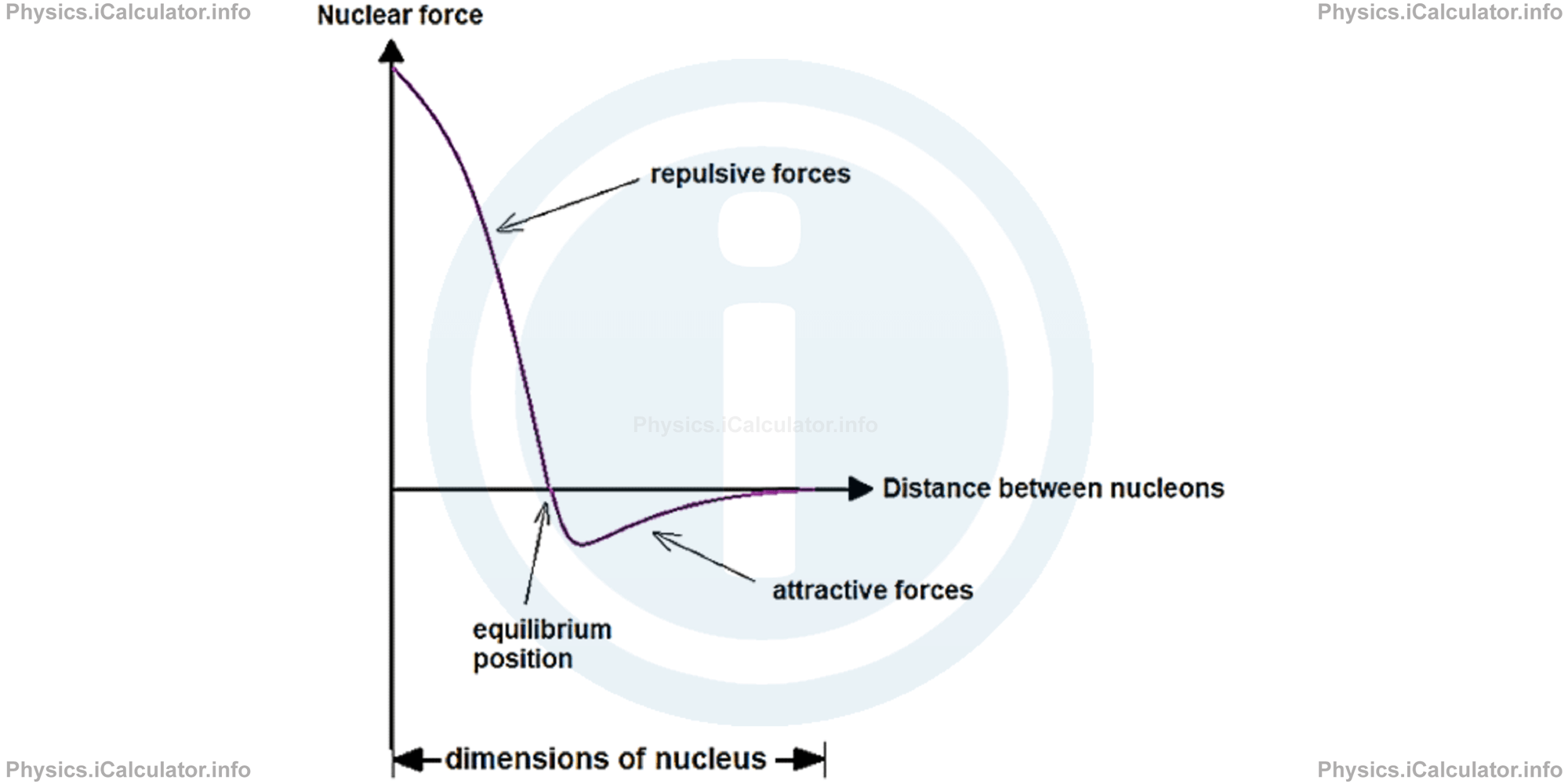
The effect of (repelling) electric force increases with the increase in the atomic number Z. For this reason, elements heavier than Uranium (Z > 92) manifest lack of stability in their nuclei and tend to break down in various ways. In those nuclei with such a high number of protons, the electric repulsion decreases less than nuclear force with the increase in distance compared to lighter nuclei. As a result, a given proton - while experiencing the electric repulsion caused by all the other protons in the nucleus - can experience the nuclear attraction caused only by the adjacent nuclei. Hence, the nucleus becomes unstable, as the proton tends to leave it.
Obviously, this approach is too simplified; the stability of nuclei depends on many other factors as well. The quantum analysis of all phenomena occurring inside atomic nuclei proves that the above rule of stability is not always valid; there are some heavier nuclei (Z = 114) in specific conditions that can be stable too. The main drawback of the above explanation on nuclear force consists on the fact that it cannot provide answer to a question that can naturally arise in everybody's mind: "Giving that one proton and one neutron are able to create a stable system (for example in hydrogen atoms), why two neutrons cannot create a system with such a stability?" This looks quite strange, given that two neutrons do not exert any electric force between them, as both of them are neutral. This question tells us that the properties of nuclear forces are more complex than we may think and they do not depend only from distance. Other factors such as the type of nucleon, orientation of spin (net nuclear angular momentum) etc., affect the magnitude of nuclear forces and the behavior of nucleons in general.
Since nucleons always have a certain non-zero distance between them (see the graph above), their interaction occurs in distance, that is, it takes place by means of distant forces (we already know that electric force is a force that acts in distance). As such, the nuclear interaction occurs not through direct contact between nucleons but by means of fields and their corresponding carriers. We will see in Section 21 that these carriers are some elementary particles known as quarks, which are smaller than protons themselves (quarks are particles that carry fractional electric charges). In fact, a single proton contains a certain number of quarks. Moreover, other elementary particles such as mesons are involved in holding the nucleons together. Therefore, it is evident that the more we delve into the study of the nucleus, the more we realize that our initial concept consisting in the consideration of atom as the smallest particle of matter was wrong; it was too far from the truth. The number of such elementary particles that are smaller than protons, neutrons and electrons is too large that nowadays there are special tables designated with the purpose to classify them, similarly to classification of elements in the periodic table.
You have reached the end of Physics lesson 20.2.1 Nuclear Force. There are 3 lessons in this physics tutorial covering Nuclear Forces, Defect of Mass and Binding Energy, you can access all the lessons from this tutorial below.
More Nuclear Forces, Defect of Mass and Binding Energy Lessons and Learning Resources
Whats next?
Enjoy the "Nuclear Force" physics lesson? People who liked the "Nuclear Forces, Defect of Mass and Binding Energy lesson found the following resources useful:
- Explanation Feedback. Helps other - Leave a rating for this explanation (see below)
- Nuclear Physics Physics tutorial: Nuclear Forces, Defect of Mass and Binding Energy. Read the Nuclear Forces, Defect of Mass and Binding Energy physics tutorial and build your physics knowledge of Nuclear Physics
- Nuclear Physics Revision Notes: Nuclear Forces, Defect of Mass and Binding Energy. Print the notes so you can revise the key points covered in the physics tutorial for Nuclear Forces, Defect of Mass and Binding Energy
- Nuclear Physics Practice Questions: Nuclear Forces, Defect of Mass and Binding Energy. Test and improve your knowledge of Nuclear Forces, Defect of Mass and Binding Energy with example questins and answers
- Check your calculations for Nuclear Physics questions with our excellent Nuclear Physics calculators which contain full equations and calculations clearly displayed line by line. See the Nuclear Physics Calculators by iCalculator™ below.
- Continuing learning nuclear physics - read our next physics tutorial: Radioactivity and Half-Life
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Nuclear Forces, Defect of Mass and Binding Energy" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.