Menu
The Kinetic Theory of Gases. Ideal Gases Practice Questions
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
The following physics revision questions are provided in support of the physics tutorial on The Kinetic Theory of Gases. Ideal Gases. In addition to this tutorial, we also provide revision notes, a video tutorial, revision questions on this page (which allow you to check your understanding of the topic) and calculators which provide full, step by step calculations for each of the formula in the The Kinetic Theory of Gases. Ideal Gases tutorials. The The Kinetic Theory of Gases. Ideal Gases calculators are particularly useful for ensuring your step-by-step calculations are correct as well as ensuring your final result is accurate.
Not sure on some or part of the The Kinetic Theory of Gases. Ideal Gases questions? Review the tutorials and learning material for The Kinetic Theory of Gases. Ideal Gases
| Tutorial ID | Title | Tutorial | Video Tutorial | Revision Notes | Revision Questions | |
|---|---|---|---|---|---|---|
| 13.6 | The Kinetic Theory of Gases. Ideal Gases |
Physics Revision Questions for The Kinetic Theory of Gases. Ideal Gases
1) 2 moles of an ideal gas expand from 3 L to 12 L when increasing its temperature from 300 K to T2 in standard atmospheric pressure. What is the final temperature of gas to the nearest unit?
- 1200 K
- 900 K
- 354 K
- 327 K
Correct Answer: C
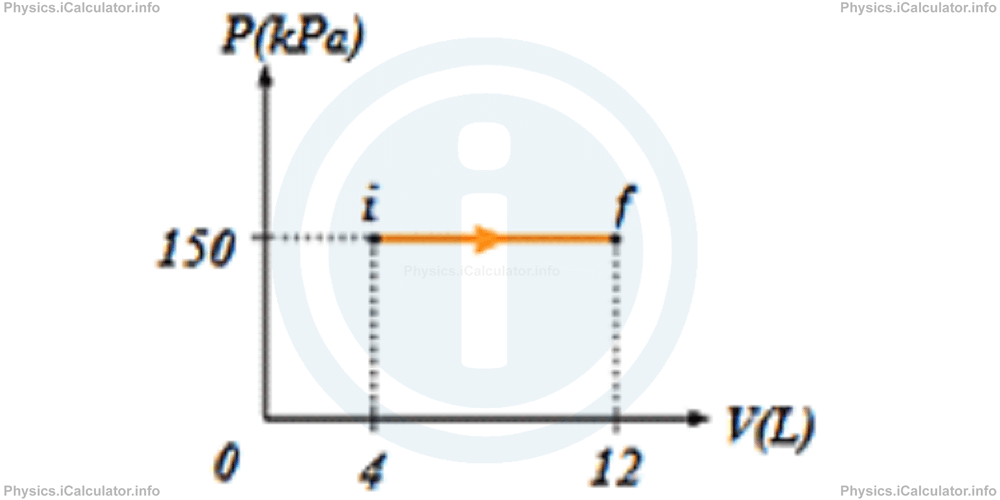
2) A gas sample expands as shown in the P - V graph below. What is the work done by the gas during expansion?
- 4800 J
- 3600 J
- 2400 J
- 1200 J
Correct Answer: B
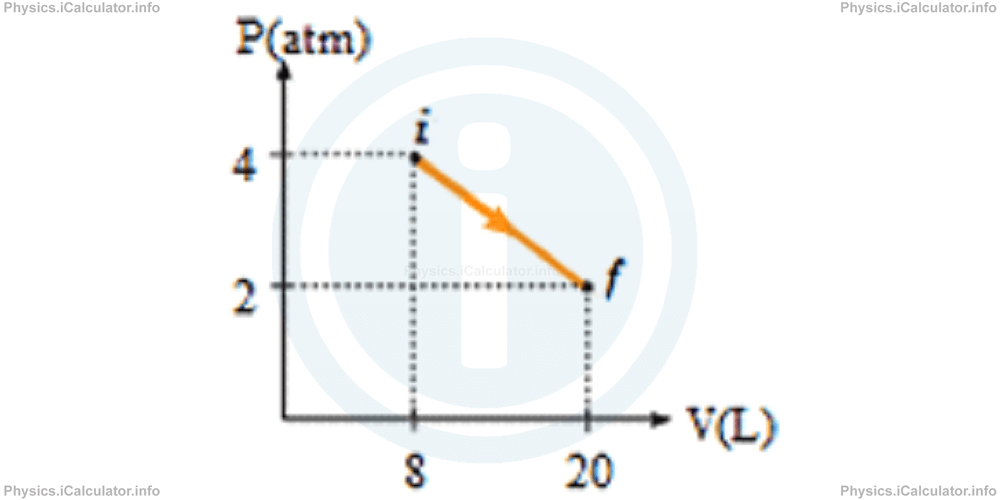
3) The P - V graph for 0.5 moles of an ideal gas is shown in the figure below. Determine the initial and final temperatures of the gas to the nearest Kelvin degree.
- Ti = 144 K and Tf = 433 K
- Ti = 433 K and Tf = 144 K
- Ti = 600 K and Tf = 1200 K
- Ti = 576 K and Tf = 1732 K
Correct Answer: A
Whats next?
Enjoy the "The Kinetic Theory of Gases. Ideal Gases" practice questions? People who liked the "The Kinetic Theory of Gases. Ideal Gases" practice questions found the following resources useful:
- Practice Questions Feedback. Helps other - Leave a rating for this practice questions (see below)
- Thermodynamics Physics tutorial: The Kinetic Theory of Gases. Ideal Gases. Read the The Kinetic Theory of Gases. Ideal Gases physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: The Kinetic Theory of Gases. Ideal Gases. Print the notes so you can revise the key points covered in the physics tutorial for The Kinetic Theory of Gases. Ideal Gases
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Pressure, Temperature and RMS Speed
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics tutorial "The Kinetic Theory of Gases. Ideal Gases" useful. If you did it would be great if you could spare the time to rate this physics tutorial (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator